Recently, faculty members from the School of Energy and Power Engineering published their latest research in the top international chemistry journal Angewandte Chemie, titled “Asymmetric Coordination in Cobalt Single-atom Catalysts Enables Fast Charge Dynamics and Hierarchical Active Sites for Two-stage Kinetics in Photodegradation of Organic Pollutants.” Jiangsu University is the first institution of completion for this paper, with PhD student Liu Xiaoming from the Barcelona Institute of Materials Science as the first author. Faculty member Wang Shuaijun from the School of Energy and Power Engineering and Lecturer Zhang Qiang from the University of Western Australia are co-corresponding authors.
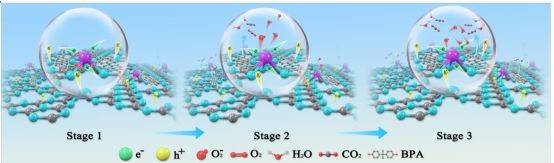
Single-atom catalysts have attracted great attention in the field of catalysis due to their 100% atomic utilization efficiency. However, traditional symmetric configurations (such as M-N4) suffer from limited electron transfer efficiency due to insufficient built-in electric field strength. Moreover, the spatial heterogeneity of light absorption, carrier localization effects, and the complex coupling of multiple active sites during photocatalytic processes further restrict the improvement of catalytic performance. Addressing these challenges, the research innovatively designed and constructed a cobalt single-atom modified carbon nitride catalyst featuring both “asymmetric coordination” and “multiple active sites.” Under visible light irradiation (λ≥420 nm), the catalyst can efficiently degrade bisphenol A within 60 minutes, achieving a removal rate of up to 98.6%, and the secondary reaction rate constant is 114 times higher than that of pure carbon nitride catalysts. Continuous flow reaction tests showed that the catalyst maintained a degradation efficiency of over 90% during 10 hours of continuous operation, demonstrating excellent stability and industrial application potential.
This study successfully achieved a breakthrough in the charge separation efficiency of the catalyst and established a two-stage kinetics mechanism for pollutant degradation. Combined with in situ X-ray photoelectron spectroscopy (XPS), in situ infrared spectroscopy (DRIFTS), and theoretical calculations, the reaction mechanism driven by asymmetric active centers was thoroughly elucidated. This work clarifies the fundamental law of how coordination symmetry regulates the electron structure of catalysts at the atomic scale and establishes a causal chain of "structural asymmetry–carrier behavior–reaction kinetics," providing a theoretical framework and technical paradigm for designing novel environmental catalytic materials. (School of Energy and Power Engineering)
Original link: https://doi.org/10.1002/ange.202507028